By: Mohammad Ahrar, PhD
Introduction
The human body consists of trillions of cells. Inside each cell, many chemical reactions occur per second and at all times to produce energy and other compounds to sustain life. Oxidation reactions are part of normal metabolic reactions in cells. However, during the process, many unstable chemicals—referred to as free radicals—are formed, which are linked to a range of disorders including certain cancers. In this issue of Peyk, we will discuss how oxidants can disrupt normal chemical reactions, and how antioxidants in foods can prevent the damaging effects of oxidants.
What is oxidation?
All elements are composed of atoms, such as oxygen, hydrogen, and nitrogen, to name a few. Each atom contains electrons that move in orbit(s) around its central nucleus. Two or more atoms combine and form a molecule. When an atom loses an electron, it becomes oxidized and the atom that steals the electron from another atom is called an oxidant. Oxygen is a very strong oxidant agent that causes oxidation, yet it is essential for human life.
How do oxidants damage cells?
During normal chemical reactions that take place in all the cells of the body (such as the breakdown of sugars and fats to produce energy), one electron may split from an atom or be stolen by other atoms such as oxygen. An atom that has lost one or more electrons can become unstable and is then referred to as a free radical.
Damaging effects of free radicals-
Free radicals are molecules with an unpaired electron which make them very unstable and in search of stability. Unstable molecules can also result from exposure to environmental chemicals (such as cigarette smoke and air pollution), or even from the damaging effects of the sun’s ultraviolet rays (2).
The problem arises when free radicals form a chain reaction: once a free radical is formed, it pulls or steals an electron from another molecule, destabilizes it, and turns it into a free radical; the new, unstable molecule then takes an electron from another molecule, destabilizing it and turning it into another free radical. This domino effect can eventually disrupt and damage the normal chemical reactions in the cells.
Several studies have suggested cellular damage caused by free radicals may be linked to a range of disorders including cardiovascular disease, certain cancers, emphysema, macular degeneration, Alzheimer’s disease, Parkinson’s disease, diabetes, and all inflammatory diseases, such as arthritis and lupus (2, 3).
Are all free radicles damaging?
Most free radicals are the natural byproducts of chemical processes in cells. They are also a crucial part of the immune system that attack bacteria and foreign invaders.
If free radicals overwhelm the body’s ability to regulate them, a condition known as oxidative stress occurs. Fortunately, the body has its own mechanism of preventing the chain reaction of free radicals before they damage the normal physiological functions in cells. Oxidative stress can be damaging when free radicals accumulate at a rate faster than the body can neutralize them. Oxidative stress is associated with damage of proteins, lipids, and nucleic acids such as DNA, and can trigger a number of human diseases.
Free radical forming agents
According to the Huntington’s Outreach Project for Education at Stanford University, substances that generate free radicals can be found in the food we eat, the medicines we take, the air we breathe, and the water we drink. Fried foods, alcohol, tobacco smoke, pesticides, certain drugs, industrial solvents, and air pollutants are among the substances that can produce the most free radicles.
Reports also show that some chemicals—such as hydrogen peroxide, nitric oxide radical, and ozone (O3)—are highly reactive species, and are capable of damaging DNA, proteins, carbohydrates, and lipids (4).
How antioxidants work
Antioxidants are molecules that can interact with and stabilize free radicals by donating an electron to neutralize them and prevent them from stealing electrons from other molecules. These low-molecular-weight antioxidants can safely interact with free radicals and stop the chain reactions before vital and normal molecules are damaged. In other words, antioxidants block harmful chemical reactions caused by oxidation. They can also bind with oxygen quickly and prevent it from oxidizing other molecules.
Antioxidants are quite stable molecules. When they donate an electron to a free radical, they will not be destabilized themselves and they stay active, thus stopping the free radical chain reactions, which in turn prevents oxidative stress in the cells that contribute to chronic diseases.
Our bodies produce some antioxidants on their own, but in insufficient amounts. Antioxidants produced in the body are part of our natural defense system to neutralize free radicals and stop them from damaging cells. For example, compounds with antioxidant properties like glutathione, ubiquinol, and uric acid are produced during normal metabolism in the body. However, the human body cannot produce enough antioxidants to cope with oxidative stress, resulting in a need to intake foods that are rich in antioxidants.
Foods high in antioxidants
Most antioxidants are found in fruits and vegetables and are collectively referred to as phytochemicals (phyto = plant). Phytochemicals give plants their vibrant colors and act as antioxidants in the body.
The antioxidant content of common fruits is listed in Table 1 (the values are milligram per 100 grams).
Table 1 Antioxidant content of common fruits
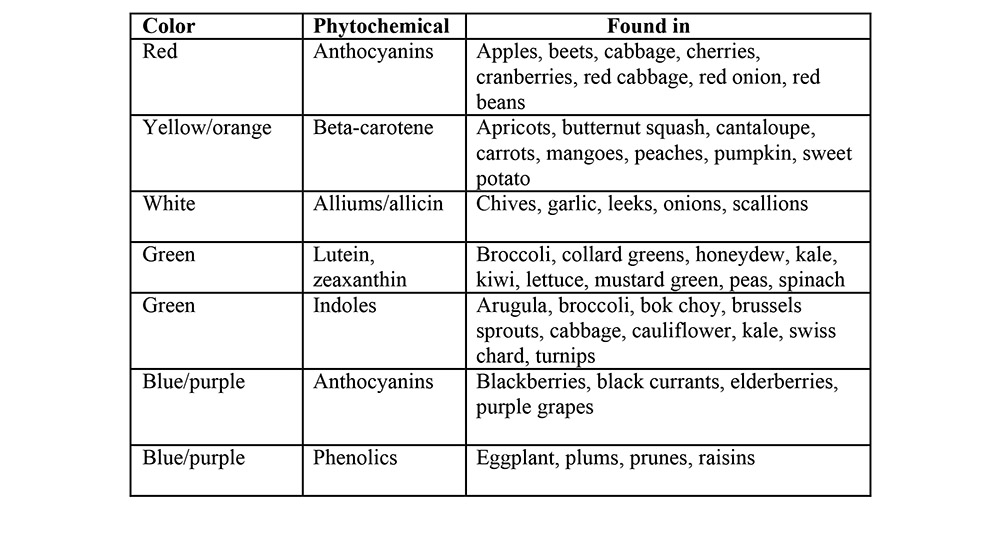
It is worth mentioning that Iranian diets include lot of fruits and vegetables that are high in antioxidants. For example, pomegranates, oranges, plums, walnuts, strawberries, dates, tea, and various green vegetables which are among the high antioxidant-containing foods are usually present on most Iranian tables.
Well-known antioxidants include beta-carotene and other carotenoids, lutein, resveratrol, lycopene, vitamin A, vitamin C, vitamin E, and other phytochemicals. The antioxidants carotenoids and flavonoids are part of a larger group of phytochemicals naturally occurring in plants and have many beneficial functions in the body. Beside their antioxidant properties, they also stimulate the immune system and interact with hormones that may help prevent certain cancers.
The presence of phytochemicals based on the color they produce has been studied and the results of some of the studies on natural antioxidants in fruits and vegetables are listed in Table 2.
Table 2- The phytochemical color guide (2)
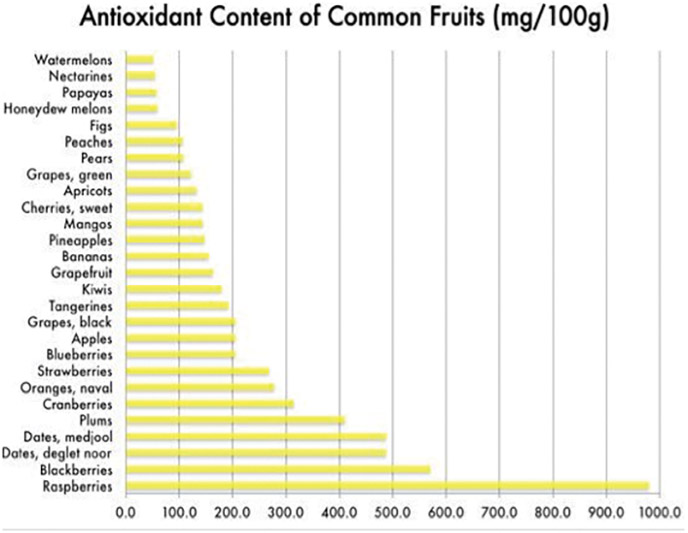
The National Cancer Institute recommends eating a variety of colorful fruits and vegetables.
The antioxidant properties of vitamin A, vitamin E, and vitamin C (not listed in Table 2) were discussed in Peyk ##169, 171, and 173. The common sources of B-carotene (provitamin A), vitamin C (ascorbic acid), and vitamin E (α-tocopherol) are summarized below:
Beta-carotene and other carotenoids – plentiful in apricots, asparagus, beets, broccoli, cantaloupe, carrots, corn, green peppers, kale, mangoes, turnip and collard greens, nectarines, peaches, pink grapefruit, pumpkin, squash, spinach, sweet potato, tangerines, tomatoes, and watermelon.
Vitamin C – abundant in berries, broccoli, Brussels sprouts, cantaloupe, cauliflower, grapefruit, honeydew, kale, kiwi, mango, nectarine, orange, papaya, snow peas, sweet potato, strawberries, tomatoes, and red, green, or yellow peppers.
Vitamin E– abundant in broccoli (boiled), avocado, chard, mustard and turnip greens, mangoes, nuts, papaya, pumpkin, red peppers, spinach (boiled), and sunflower seeds.
Do all antioxidants have the same effectiveness?
Although some foods may contain more antioxidants than others, the effectiveness and strength of antioxidants is also of a question. For example, two different foods may have the same amount—but different types—of antioxidants. Many studies have been focused on the strength of different antioxidants. The results of the effectiveness of antioxidants in some common foods in some studies are shown in Table 3. The values are based on Oxygen Radical Absorbance Capacity (ORAC). The higher the value, the stronger and more effective an antioxidant will be.
Table 3- Effectiveness of different antioxidants in some common foods

The National Cancer Institute recommends eating a variety of colorful fruits and vegetables daily to provide your body with valuable vitamins, minerals, fiber, and disease-fighting phytochemicals.
Summary
During the cellular metabolism process that breaks down fats, carbohydrates, and other substances, many unstable chemicals, referred to as free radicals, are formed. If these unstable molecules overwhelm the body’s ability to regulate them, a condition known as oxidative stress occurs, and can result in many disorders including certain cancers.
Antioxidants are molecules that can interact with and stabilize free radicals by donating an electron to neutralize them and prevent them from stealing electrons from other molecules, therefore blocking the harmful chemical reactions caused by oxidation.
Antioxidants are found in fruits and vegetables, and are collectively referred to as phytochemicals, compounds that give fruits and vegetables their vibrant colors.
References;
1-Modern Nutrition in Health and Disease, Maurice E. Shills, et. al., 10th ed. Lippincott, Williams and Wilking.
2-Nutrition: From Science to You, Joan Salge Blake, Kathy D Munoz, and Stella Volpe- 3rd ed. 2016, Pearson Education, Inc.
3-https://www.livescience.com/54901-free-radicals.html
4-https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3249911/
5-https://www.webmd.com/food-recipes/antioxidants-your-immune-system-super-foods-optimal-health
6-https://www.tetonhospital.org/documents/cognitive-health/top-20-foods-high-in-antioxidants.pdf


















